DTB provides comprehensive testing, engineering, and technical documentation services to ensure the performance, reliability, and compliance of medical devices. Our expert team helps manufacturers meet the stringent regulatory requirements necessary to bring life-saving technology to market. From environmental and electromagnetic testing to technical publications, DTB is a trusted partner for the medical industry.
Medical devices must adhere to strict regulatory and quality standards to ensure safety and effectiveness. DTB’s expertise in medical device compliance helps manufacturers navigate these complex requirements, providing thorough testing and validation.
Key standards and certifications we adhere to include:
for medical electrical equipment safety
for medical device quality system regulations
for electromagnetic compatibility in medical devices
for quality management systems in medical device manufacturing
for risk management in medical device development
DTB ensures that medical products meet or exceed industry requirements for safety, performance, and regulatory compliance.
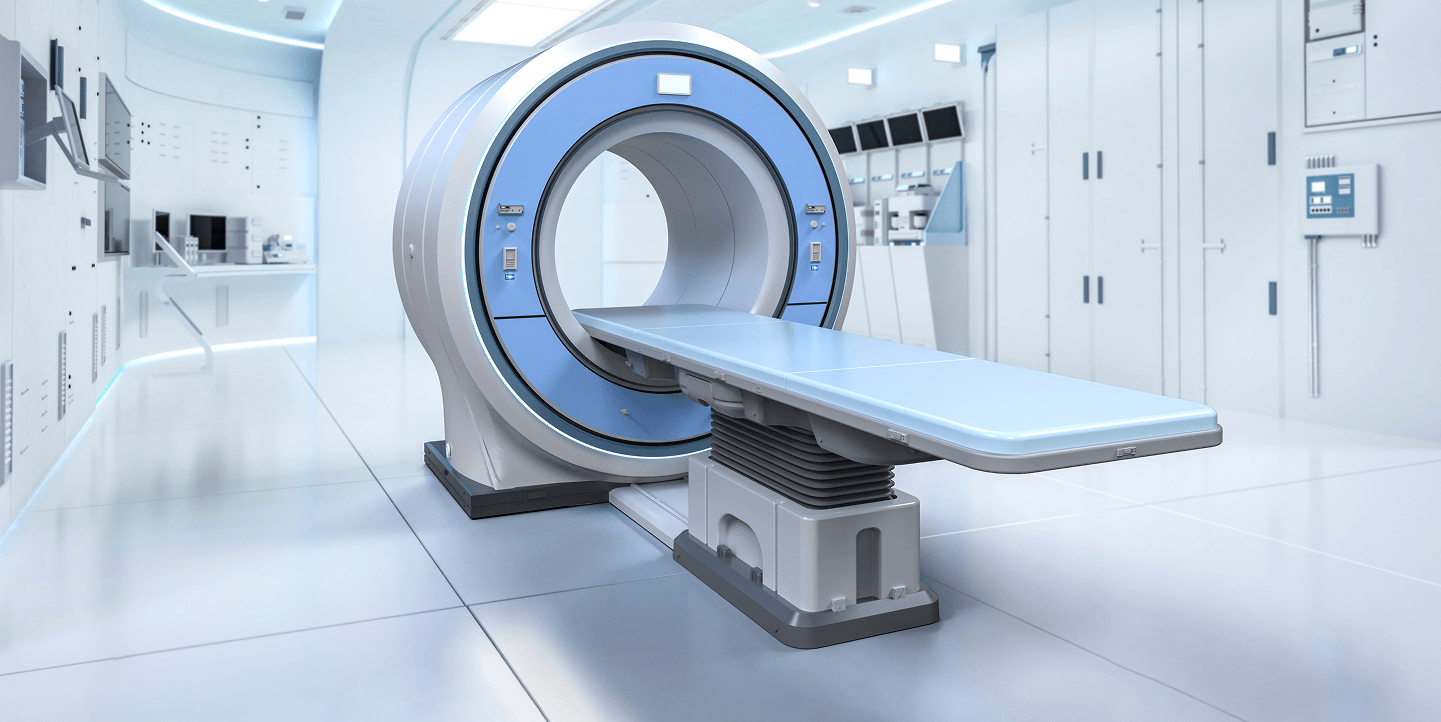
Medical devices must perform flawlessly in a variety of environments, often under extreme or unpredictable conditions. DTB provides state-of-the-art testing solutions to ensure compliance, durability, and performance from conceptualization through production.
Our engineering team works closely with clients to “design in” reliability, identifying potential failure modes early in development. For electronic devices, we validate EMI/EMC compliance at the prototype stage, helping to prevent costly redesigns. Additionally, we conduct full design verification testing to ensure regulatory approval and market readiness.
Assessing electromagnetic compatibility and interference to meet global standards.
Simulating real-world handling, transport, and operational conditions.
Testing for temperature extremes, humidity, voltage fluctuations, and power supply variations.
Ensuring long-term reliability under continuous operation.
Evaluating structural integrity for portable and handheld medical devices.
Validating compliance with international safety regulations.
Assessing susceptibility to electrostatic shocks.
Measuring potential interference with sensitive medical equipment.
Ensuring stable operation under power fluctuations.
Analyzing durability under repeated mechanical impacts.
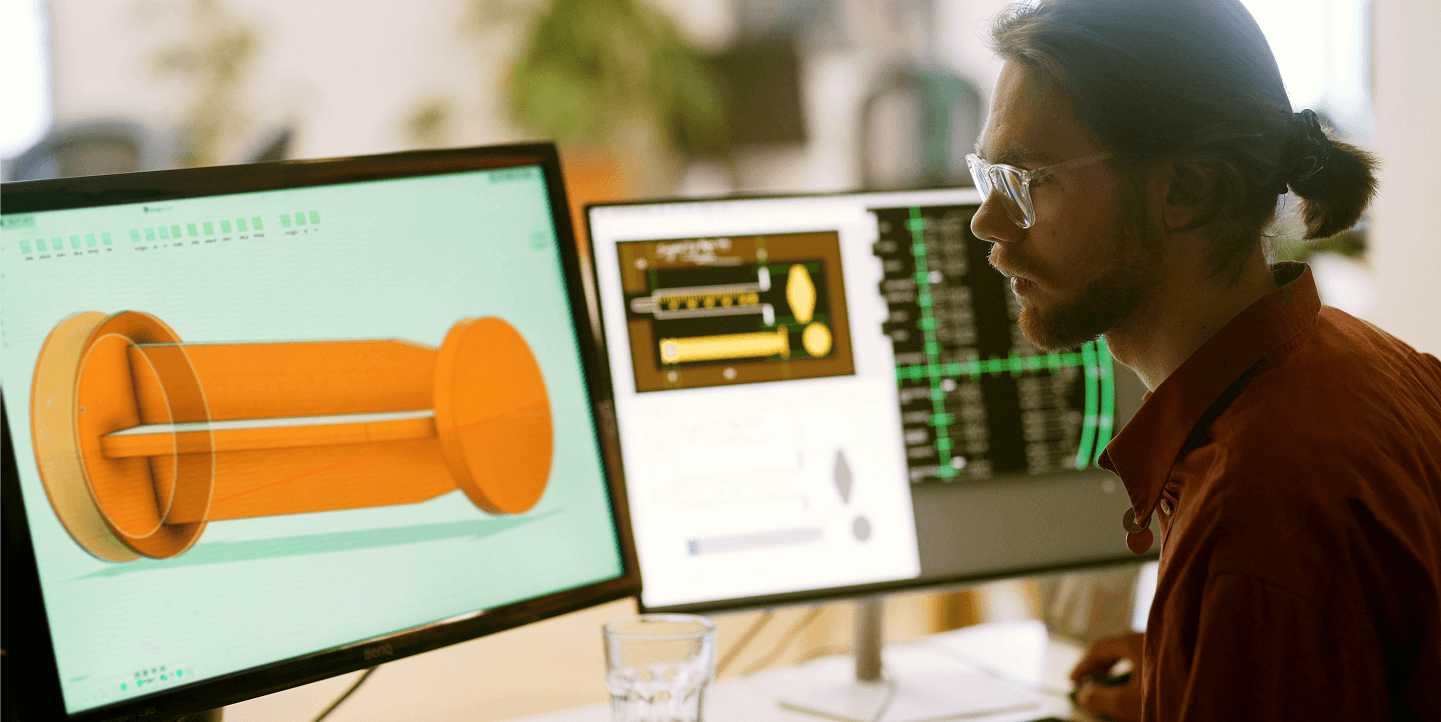
Medical device manufacturers must provide precise, compliant, and user-friendly documentation to support safe operation, maintenance, and regulatory approval. High-quality technical documentation is essential for healthcare professionals, biomedical engineers, and regulatory bodies. DTB’s expertise in technical publications ensures ensures your products are fully supported with comprehensive and compliant documentation.
Clear, concise guides for end-users, technicians, and biomedical engineers.
Ensuring adherence to FDA 21 CFR Part 11, ISO 13485, and IEC 62366 standards.
Developing instructional materials for healthcare professionals and maintenance staff.
Providing detailed reference materials for servicing and repairs.
Aligning documentation with industry best practices for safety and compliance.
In healthcare, properly structured and professionally designed documentation is critical—not only for regulatory approval but also for the seamless integration of medical equipment into hospital and clinical environments. Comprehensive equipment information systems, including digital and printed manuals, must deliver accurate operating and maintenance procedures on demand. These systems often play a key role in a purchasing decision, as hospitals and healthcare providers prioritize reliability, ease of use, and ongoing support.
DTB’s end-to-end documentation solutions ensure that your medical devices are backed by the highest standard of technical publications, improving usability, compliance, and market acceptance.
DTB provides industry-leading expertise in medical device testing, engineering, and documentation. Our state-of-the-art facilities, regulatory knowledge, and experienced team make us the ideal partner for manufacturers seeking compliance, reliability, and efficiency.

Decades of experience in regulatory testing and validation.
Custom test protocols designed for your specific device requirements.
Cutting-edge testing environments for EMC, vibration, environmental, and performance evaluations.
From initial testing to documentation and lifecycle management, we provide end-to-end solutions.
Trusted by medical manufacturers to ensure product safety and reliability.
From hospital operating rooms to emergency medical services, DTB-tested and documented devices are helping save lives worldwide.
Contact DTB today to learn how we can support your medical device program with tailored solutions and proven expertise.
Dayton T. Brown, Inc. 1175 Church St. Bohemia, NY 11716
Dayton T. Brown, Inc. 23967 Prop Way Hollywood, MD 20636